About 10% of the children with alpha1-antitrypsin deficiency must undergo liver transplantation. Their hepatocytes fail to properly degrade polymers of mutant alpha1-antitrypsin (ATZ), whose accumulation is highly toxic to cells.
In these patients, elusive genetic and/or environmental modifiers contribute to the harmful progression of the disease caused by the Z mutation, thus resulting in dramatic consequences.
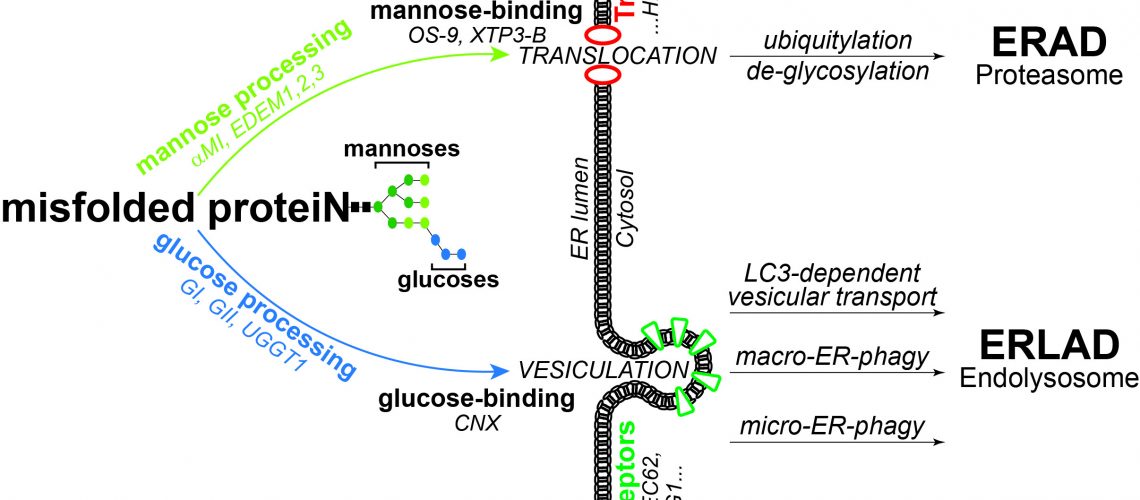
Supported by the Alpha-1 Foundation (US), Molinari’s lab is investigating the mechanisms of clearance from cells of disease-causing ATZ polymers, with the understanding that these mechanisms might be defective in liver disease associated to alpha1-antitrypsin deficiency. The lab recently reported that large misfolded proteins such as ATZ polymers (Fregno et al. EMBO J 2018) and mutant forms of pro-collagen (Forrester et al. EMBO J 2019) are segregated in sub-domains of the ER that are eventually delivered to lysosomes for clearance. These degradative pathways are engaged by misfolded species that cannot be degraded by cytosolic proteasomes and have been collectively named ER-to-Lysosome-Associated Degradation (ERLAD). ERLAD relies on ER-phagy receptors (e.g., FAM134B) that engage components of the autophagic machinery at the ER membrane. This promotes the formation of ER-derived vesicles that contain toxic material and their delivery to the lysosomal compartment for destruction.
The current paper (Fregno et al EMBO J 2021) identifies the signal that channels misfolded proteins in the ERLAD pathway. It consists in cycles of removal/re-addition of glucose residues from protein-bound oligosaccharides driven by the ER-resident a-glucosidase I and a-glucosidase II and by the quality control factor UGGT1. These cycles result in persistent association of the glucose-binding lectin calnexin with the misfolded species. Calnexin forms a functional complex with the ER-phagy receptor FAM134B, an event that eventually channels misfolded polypeptides in the ERLAD pathway. The data reinforce the concept that processing of oligosaccharides that are co-translationally added onto newly synthesized polypeptides plays a crucial role in quality control of the eukaryotic cell’s proteome. As shown in the figure, mannose processing selects misfolded proteins for proteasomal clearance (ER-associated degradation, ERAD), glucose processing selects proteasome-resistant polypeptides for ERLAD.
Article
Fregno I, Fasana E, Soldà T, Galli C, Molinari M. EMBO J. 2021 Jun 21:e107240. doi: 10.15252/embj.202010724